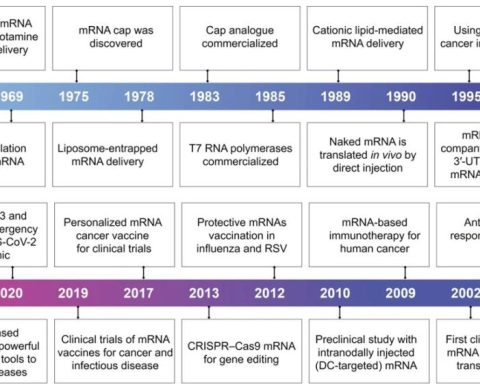
The Value of RWE in the Product Lifecycle for Emerging Biopharmaceutical Companies
By Barbara Arone, Vice President, Real World Solutions, IQVIA The use of real-world evidence (RWE) in the development of pharmaceutical treatments has grown exponentially in the past 10 years, fueled by availability of higher quality data, trial competitiveness and growing investments in personalized and