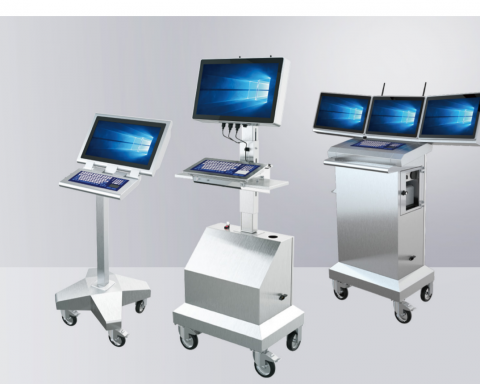
The future of pharmaceutical manufacturing is mobile. Advances in cloud computing and the onset of Industry 4.0 has led to the proliferation of mobile IT solutions for manufacturing, including pharmaceuticals. Particularly, mobile workstations and tablets are becoming staples of the pharmaceutical manufacturing workflow due to their cost-effectiveness, particularly in demanding environments like cleanrooms, where even modular fixed human-machine interfaces (HMIs) can be exorbitantly priced and costly to repair, not even factoring in the hidden costs (e.g., ergonomics). However, it can be difficult to determine what separates great mobile workstations from those that merely allow you to get on with your work. This article will highlight some of those distinctions.
How Mobile is it?
Built-in HMIs and workstations are expensive. Beyond the considerable cost of installation—hiring experts to assess the viability of installation, fixing mounts in place on walls or ceilings, and connecting them to internal cabling, not to mention shutting down and cleaning the cleanroom in which they are installed—they are far less flexible in terms of usage and workflow, and far more difficult to replace. Moreover, at least one must be installed at each station in the laboratory in order to be viable.
Mobile workstations and HMIs, by contrast, are cheaper. There are no installation costs; they can generally be used from the get-go after unboxing and onboarding of the PC/client. They can also replace multiple fixed HMIs, as they can be moved between stations. Blue Line, an HMI manufacturer, sums up the affordances of mobile solutions quite nicely: “Reduce both installation and manufacturing costs and obtain a flexible position for shifts in production types and batches.” 1
However, their defining characteristic—their mobility—must extend beyond the basics of moving smoothly on horizontal surfaces and remaining upwardly stable on inclines even at full extension (they must at least be designed to exceed the exacting mechanical stability and safety standards of the IEC 60601-1 3rd Edition).
Operating time is particularly important: mobile operating stations must be able to be fully operational and wireless for at least long as a shift. Workstations that rely on built-in batteries may often fail at this task, especially as their batteries degrade through repeated usage. Hot-swappable battery systems—where the batteries can be removed and charged externally to allow for quick switching and no downtime between shifts—excel here, especially in cleanrooms, where space is at premium and uptime is essential.
Workstations containing space for client PCs as well as multiple peripherals also excel in pharmaceutical environments, allowing workers to move throughout the cleanroom with all the equipment necessary to complete their tasks. Collating this equipment in a single, mobile space limits clutter on work surfaces throughout the laboratory, making cleaning easier as well as reducing the possibility of these peripherals being exposed to harmful substances. Moreover, clients can be directly connected to the workstation, eliminating KVM extenders’ need and ensuring overall greater flexibility.
Of course, onsite mobility is only half the picture. Many pharmaceutical companies operate across national borders, and workers may have to visit many sites to maintain production continuity. In doing so, they may have to adapt to several different HMIs, which can inhibit workflow. Moreover, with the growing trend in personalized medicine, smaller-scale pharmaceutical production is becoming more common. Accordingly, smaller-scale, more temporary cleanrooms are becoming more fashionable; however, these may lack a fixed HMI or have high competition for charging stations. This is where hot-swappable battery functions shine—such workstations can be transported and immediately set up with a fully charged battery to allow no loss of downtime. In these cases, of course, it’s necessary to partner with a manufacturer that offers consulting on logistics and can quickly offer support when the inevitable configuration problems arise.
How Durable and Hygienic Is It?
Mobility is a definite asset to pharmaceutical laboratories, but it can also pose some issues in sterile environments. Their flexibility means that they can be exposed to a much greater variety of corrosive substances and contaminating agents; as such, they should be designed with cleanability in mind. They should be made of materials that can weather the most caustic cleaning agents and hazardous substances, such as 316L stainless steel (a low-carbon alloy resistant to corrosion), preferably electropolished to reduce the number of micro-crevices in which bacteria and other air-quality contaminants can hide. Workstations should be tested extensively for their ability to weather chemicals; ideally, they should receive an “excellent” rating on the SEFA 24-hour exposure test for the common chemicals and substances in a given laboratory environment. The material should show little to no change after 24-hour exposure to a substance to receive an excellent rating. Such durability ensures that they can endure even the most rigorous cleaning procedures across all ISO 14644 cleanroom classes.
Mobile HMIs should also possess solid ingress protection (at least IP65)—that is, they should be waterproof—allow for thorough and frequent washing and cleaning. They should also have minimal horizontal surfaces, which become dirtier far more quickly than vertical surfaces, and smaller overall surface areas so that cleaning them is quick and efficient. Cleanability is particularly important for workstations that travel between worksites with different cleanroom classes. Workstations should be usable while wearing personal protective equipment, even touchscreen HMIs, to limit their exposure to human-borne contaminants.
How Ergonomic is It?
Mobility also allows for better ergonomics, the impact of which is considerable. Muskuloskeletal disorders (MSDs) are a rampant problem in industrial settings, as workers tend to remain in the same position and may need to use devices not suited to their height or build, leading to strain on the back, shoulders, and neck. There is considerable evidence that ergonomic interventions reduce the likelihood of MSDs2, which in turn translates to fewer disability claims and worker sickness absences. Consideration of ergonomics has considerable cost-saving benefits for manufacturers (see the systematic review by Tompa et al.3) and can increase worker well-being and productivity.
Accordingly, good mobile workstations should have a range of positional settings. HMIs and work surfaces should have stepless height adjustment ranges of at least 20 cm. The adjustment can be manual or electric (or some combination). Swivel (±60º) and tilt (-5º to over +20º) capabilities are a plus. These features ensure that workers can work sitting or standing (preferably a mix of both) in a neutral posture throughout the day, leading to reduced musculoskeletal strain and therefore a reduced likelihood of repetitive stress injury.
Moreover, peripherals should be storable at a manageable height for most workers to ensure smooth retrieval. Power switches, cable hooks, and batteries should be easily reachable without undue strain. Although these may seem like minor issues, they add up to have a big impact on workers, who often have to these workstations for hours on end.
Conclusion
Mobile workstations afford pharmaceutical workers the ability to conduct their lifesaving and wellbeing-enhancing work in a flexible and ergonomically supportive manner. These features ultimately translate to safer production conditions (and therefore safer products), greater worker satisfaction, and reduced costs. As such, pharma manufacturers would do well to seek out these mobile IT solutions as soon as possible.
References
1. https://www.blue-line.com/products/mobile-operator-station
2. Rivilis I, Van Eerd D, Cullen K, et al. Effectiveness of participatory ergonomic interventions on health outcomes: a systematic review. Appl Ergon. 2008;39(3):342-358. doi:10.1016/j.apergo.2007.08.006
3. Tompa E, Dolinschi R, de Oliveira C, Amick BC, Irvin E. A Systematic Review of Workplace Ergonomic Interventions with Economic Analyses. J Occup Rehabil. 2010;20(2):220-234. doi:10.1007/s10926-009-9210-3






what are the pros and cons of mobile workstations?